iPhone Urinalysis App Draws U.S. Government Scrutiny
The U.S. Food and Drug Administration has sent a letter to BioSense Technologies over its iPhone uChek urinalysis system, asking why its medical app hasn't been cleared by the agency. The app is one of the first that turns the iPhone into a medical device, designed to read urinalysis test strips that are normally examined by users and compared to a color-coded chart.
With the uChek system, patients can take a picture of the strip with the iPhone's camera and then receive an automated readout of parameters like glucose, urobilinogen, pH, ketone and more. The app also stores results which then can be analyzed over time.
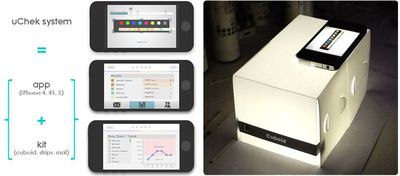
Though medical device makers have adopted the iPhone for some measurements like blood glucose monitoring for diabetics, large scale use of smartphones and tablets as a replacement for existing medical devices has yet to take off -- likely due in large part to government regulation of medical devices.
From Bloomberg:
Biosense Technologies Private Ltd.’s uChek system isn’t cleared by the Food and Drug Administration and the agency said it wants to know why not, in a first-of-its-kind letter to a maker of a mobile-device application. The app relies on users, such as diabetics checking their glucose, to dip test strips in urine and use the smartphone’s camera to allow the system to processes and generate automated results.
UChek works with test strips made by Siemens AG (SIE) and Bayer AG (BAYN), which are only approved for visual reading and require new clearance for automated analysis, the FDA said in the letter. The agency has said it wants stricter rules for apps that directly diagnose or treat conditions, proposing in 2011 to apply similar quality standards as for heart stents, ultrasound machines and other medical devices.
The uChek kit can be purchased in the US and India for $40, while the uCheck iPhone app is a free download [Direct Link] from the App Store -- though the app can also manually read urine strips from other companies.
Popular Stories
Last year, Apple launched CarPlay Ultra, the long-awaited next-generation version of its CarPlay software system for vehicles. Nearly nine months later, CarPlay Ultra is still limited to Aston Martin's latest luxury vehicles, but that should change fairly soon.
In May 2025, Apple said many other vehicle brands planned to offer CarPlay Ultra, including Hyundai, Kia, and Genesis.
In his Powe...
The calendar has turned to February, and a new report indicates that Apple's next product launch is "imminent," in the form of new MacBook Pro models.
"All signs point to an imminent launch of next-generation MacBook Pros that retain the current form factor but deliver faster chips," Bloomberg's Mark Gurman said on Sunday. "I'm told the new models — code-named J714 and J716 — are slated...
Apple is planning to launch new MacBook Pro models with M5 Pro and M5 Max chips alongside macOS 26.3, according to Bloomberg's Mark Gurman.
"Apple's faster MacBook Pros are planned for the macOS 26.3 release cycle," wrote Gurman, in his Power On newsletter today.
"I'm told the new models — code-named J714 and J716 — are slated for the macOS 26.3 software cycle, which runs from...
We are still waiting for the iOS 26.3 Release Candidate to come out, so the first iOS 26.4 beta is likely still at least a week or two away. Following beta testing, iOS 26.4 will likely be released to the general public in March or April.
Below, we have recapped known or rumored iOS 26.3 and iOS 26.4 features so far.
iOS 26.3
iPhone to Android Transfer Tool
iOS 26.3 makes it easier...
In 2022, Apple introduced a new Apple Home architecture that is "more reliable and efficient," and the deadline to upgrade and avoid issues is fast approaching.
In an email this week, Apple gave customers a final reminder to upgrade their Home app by February 10, 2026. Apple says users who do not upgrade may experience issues with accessories and automations, or lose access to their smart...



















